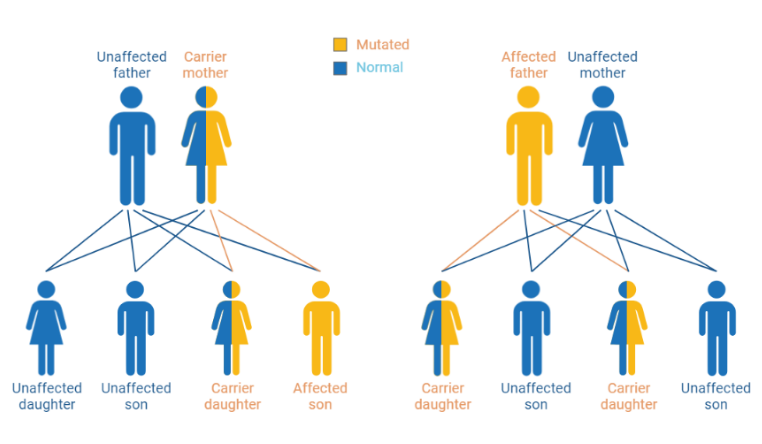
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzymopathy in the world. G6PD is the first and rate-limiting enzyme in the pentose phosphate pathway. Individuals with G6PD deficiency have a decreased ability to produce reduced nicotinamide adenine dinucleotide phosphate (NADPH), crucial in protecting red blood cells from damage caused by free radicals. The genetic disorder is caused by mutations in the G6PD gene, located on the X chromosome and inherited in an X-linked manner. As such, males are more likely to be clinically affected by G6PD deficiency if they inherit the mutated X chromosome from their mothers. Females are usually carriers of the G6PD deficiency since they inherit the X chromosome from both their mother and father.[1, 4, 5] In the case where both X-chromosomes are mutated in homozygous conditions, clinically, these females will manifest low G6PD activity as deficient males. In Indonesia, malaria-endemic areas have been noted to be prevalent for G6PD deficiency. For example, in Southwest Sumba, the prevalence of G6PD deficiency is 7%.[6] The inability to produce NADPH for the downstream glutathione cycle, an anti-oxidative pathway in the cell, makes red blood cells more vulnerable to oxidative stress, as they are less equipped to neutralize reactive oxygen species (ROS) and other harmful molecules. Thus, certain drugs or foods can act as triggers for oxidative stress and exacerbate hemolysis in individuals with G6PD deficiency.[7, 8]
Drug-induced hemolysis is the destruction of red blood cells caused by exposure to certain medications or chemicals in the spleen. Many of these compounds can be commonly found in over-the-counter drugs, prescribed drugs, and foods sold in Indonesia. Therefore, it is crucial for individuals with G6PD deficiency to be aware of potential triggers and to inform healthcare providers about their condition.[2, 3]
Are you aware that these common drugs can potentially increase hemolytic risk in G6PD-deficient patients?
Some drugs can present a definite risk of hemolysis, whilst some drugs have less risk of inducing hemolysis.
Definite risk of hemolysis:
| Drug Group | Drug or Substance | Indonesian Market/Brand Name |
|---|---|---|
| Antibiotics | Nitrofurans Class: furazolidone, nitrofurazone, furaltadone, nitrofurantoin | Nitrofurantoin, Urfadyn, Diafural, Sanfuro, Nifural |
| Quinolone Class: 4-quinolone, ciprofloxacin, levofloxacin, moxifloxacin, nalidixic acid | Cifloxan, Meflosin, Interflox, Bernoflox, Bimaflox, Kirafox, Cylowam, Ciflos, Phaproxin, Ciproxin, Tequinol, Quidex, Quinobiotic, Baquinor Forte, Bufacipro, Ciprofloxacin HCl | |
| Sulfonamide Class: sulfasalazine, sulfisoxazole, sulfamethoxazole | Cotrimoxazole, Decatrim, Infatrim, Albucetine, Albuvit 10%, Albuvit 15%, Lazafin, Sulcolon, Septra | |
| Chloramphenicol | Bufacetine, Colsancetine, Chlorexol, Denicol, Erbacetine, Hufamycetin, Kalmicetine | |
| Furazolidone | Furoxone, Dependal-M | |
| Streptomycin | Streptomycin Sulphate Meiji | |
| Antimalarial Drugs | Mepacrine – not implemented in Indonesia | Atabrine, Quinacrine, Mepalex, Acriquine, Atebrin |
| Primaquine | Primaquine Phosphate | |
| Tafenoquine – has not obtained permission for distribution in Indonesia | — | |
| Pyrimethamine | Daraprim, Primet | |
| Quinine | Quinine Hydrochloride, Quinine Sulfate, Tablet Kina | |
| Parenteral Anesthesia | Prilocaine | Takipril |
| Antimycobacterial | Dapsone | Aczone, Acnedap |
| Para-aminosalicylic Acid (PAS) | Paser | |
| Antineoplastic Adjuvant | Doxorubicin | Caelyx, Doxotil, Doxorubicin HCl, Kemodoxin, Naprodox, Sandobicin, Sindroxocin, Adriamycin, Doxil |
| Rasburicase | Elitek, Fasturtec | |
| Genitourinary Analgesic | Phenazopyridine | Nexurin, Urogetix, Pyridium, Prodium, Pyridiate, Baridium, Uricalm |
| Analgesic | Acetylsalicylic Acid | Aspirin, Ascardia, Aspilets, Astika, Bodrexin, Cardio Aspirin, Cartylo, Contrexyn, Coplavix, Farmasal, Gramasal, Inzana, Novosta, Remasal, Aspergum, Genacote, Halfprin |
| Antidiabetic | Glibenclamide | Gliben-J, Daonil, Diabeta, Euglucon, Condiabet, Velacom, Glimepix, Amadiab, Gluvas M |
| Antihypertensive | Hydralazine | Apresoline, Hylazine, Aprezin Tab, Hyperzine |
| Methyldopa | Aldomet, Dopamet 250 mg, Dopamet 500 mg, Medopa | |
| Antiviral Drugs | Probenecid | Probenid |
| Vitamin K | Phytomenadione (Vitamin K1) | Vitadion, Neo-K, Pro K, Quadion, Mepro K, Vitka Infant |
| Miscellaneous | Methylene Blue | — (injection) |
| Fava Beans | — | |
| Naphthalene | Mothballs |
Possible risk of hemolysis (safe to consume in therapeutic dose):
| Drug Group | Drug or Substance | Indonesian Market/Brand Name |
|---|---|---|
| Antimycobacterial | Isoniazid | Cultube 3 FDC Paed, Erabutol Plus, Inadoxin Forte, Inha, INH-CIBA, Inoxin, Isoniazid, Kapedoxin, Metham, Pehadoxin Forte, Pulna Forte, Pro TB, Pyravit, Rifanh, Rifastar, Rimcure Paed, Suprazid Forte, TB Vit 6 |
| Analgesic | Paracetamol | Calpol, Panadol, Naprex, Paramol, Mixagrip Flu, Hufagesic, Paramex SK, Sanmol, Sumagesic, Termorex, Poro |
| Nitroglycerin | DBL Glyceryl Trinitrate Concentrate Injection, Glyceryl Trinitrate, NTG, Nitral, dan Nitrokaf Retard | |
| Phenazone | Antipyrine, Etamidon, Otil, Blephamin, Tytin | |
| Phenylbutazone | Afitazon, Akrofen, Berlizon, Enkapyrin, Erphazon, Erzon, Etacyl, Fenilbutazon, Ifirema, Irgapan, Novason, Rheumadix, Rheumakap, Selesfen, Zerion, Zonifar | |
| Tiaprofenic Acid | Surgamyl | |
| Antihistamine | Antazoline | Vasacon-A, Ximex Tason, Ximex Zifen, Zinc Prima A |
| Diphenhydramine | Allerin Expectorant, Benadryl, Borraginol-N, Camydril, Decadryl, Dextrosin, Diphenhydramine HCL, Fortusin, Ikadryl, Iphadryl, Kontrabat, Licodril, Molexdryl, Novadryl, Otede, Recodryl, Sanadryl, Siladex DMP, Woods Peppermint Antitusive, Yekadryl, Zecadryl | |
| Tripelennamine | Pyribenzamine | |
| Antiviral Drugs | Colchicine | Recolfar, Ar-Gout, Kolsin, Nucine, Pyricin, Frigout, L-Cisin |
| Vitamin K | Menadione | Vitamin K Table Salut Gula (Kimia Farma), Katin, Keetomin, Vicasol |
| Vitamin C | Ascorbic Acid | Vitamin C supplements |
| Parenteral Anesthesia | Lidocaine | Extracaine, Lidocaine HCl Monohydrate, Lidox, Lidocom, Lidodex, Pehacain |
| Anti-Parkinson’s Disease | Trihexyphenidyl | Arkine, Artane, Hexymer, Parkinal, Trihexyphenidyl, Trihexyphenidyl HCl |
| Cardiovascular Drugs | Dopamine | Generik, Cetadop, Domia, Dopamine HCl, Proinfark, Indop 200, Udopa, Dopac |
| Procainamide | Procan, Pronestyl, Procanbid, Procapan | |
| Miscellaneous | Para-aminobenzoic acid (PABA) | Vitamin B10 supplements |
EHI actively participates in preventing drug-induced hemolysis through G6PD screenings, surveys, and socialization among target populations regarding the importance of G6PD status and awareness of hemolysis-inducing compounds in rural areas. Increasing public awareness of G6PD deficiency can help deficient individuals to be screened and healthcare providers make safe decisions on treatment. The risks associated with specific drugs must help healthcare professionals tailor treatment plans and choose alternatives to prevent drug-induced hemolysis. Along with this, it would be wise for patients with G6PD deficiency to independently verify that the medications they are consuming are not drugs mentioned in the previous tables. A list of drugs distributed in Indonesia is accessible through the Indonesian Food and Drug Authority’s library at https://cekbpom.pom.go.id/obat.
Additionally, please take a look at other online references for more information on which drugs/supplements are safe/unsafe for G6PD-deficient individuals:
- Mayo Clinic Drugs and Supplements: https://www.mayoclinic.org/drugs-supplements
- Monthly Index of Medical Specialties (MIMS) Indonesia Drug Library: https://www.mims.com/indonesia/drug
- Johns Hopkins Guides: https://www.hopkinsguides.com/hopkins
References:
[1] Carter, T. E., Mekonnen, S. K., Lopez, K., Bonnell, V., Damodaran, L., Aseffa, A., & Janies, D. A. (2018). Glucose-6-phosphate dehydrogenase deficiency genetic variants in malaria patients in Southwestern Ethiopia. The American Journal of Tropical Medicine and Hygiene, 98(1), 83. https://doi.org/10.4269%2Fajtmh.17-0557
[2] La Vieille, S., Lefebvre, D. E., Khalid, A. F., Decan, M. R., & Godefroy, S. (2019). Dietary restrictions for people with glucose-6-phosphate dehydrogenase deficiency. Nutrition Reviews, 77(2), 96-106. https://doi.org/10.1093/nutrit/nuy053
[3] Lee, S. W. H., Lai, N. M., Chaiyakunapruk, N., & Chong, D. W. K. (2017). Adverse effects of herbal or dietary supplements in G6PD deficiency: a systematic review. British Journal of Clinical Pharmacology, 83(1), 172-179. https://doi.org/10.1111%2Fbcp.12976
[4] Luzzatto, L., Ally, M., & Notaro, R. (2020). Glucose-6-phosphate dehydrogenase deficiency. Blood, The Journal of the American Society of Hematology, 136(11), 1225-1240. https://doi.org/10.1182/blood.2019000944
[5] Richardson, S. R., & O’Malley, G. F. (2022). Glucose-6-Phosphate Dehydrogenase Deficiency. In StatPearls [Internet]. StatPearls Publishing. Retrieved August 4, 2024 from https://www.ncbi.nlm.nih.gov/books/NBK470315/
[6] Satyagraha, A. W., Sadhewa, A., Baramuli, V., Elvira, R., Ridenour, C, Elyazar, I., Noviyanti, R., Courtier F. N., Harahap, A. R., Baird, J. K. (2015). G6PD Deficiency at Sumba in Eastern Indonesia is Prevalent, Diverse and Severe: Implications for Primaquine Therapy against Relapsing Vivax Malaria. PLoS NTD, 9(3), e0003502. https://doi.org/10.1371/journal.pntd.0003602
[7] Stanton, R. C. (2012). Glucose‐6‐phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life, 64(5), 362-369. https://doi.org/10.1002%2Fiub.1017
[8] Yang, Y., Li, Z., Nan, P., & Zhang, X. (2011). Drug-induced glucose-6-phosphate dehydrogenase deficiency-related hemolysis risk assessment. Computational Biology and Chemistry, 35(3), 189-192. https://doi.org/10.1016/j.compbiolchem.2011.04.010






